BJMO - volume 19, issue 4, june 2025
E. Oldenburger MD, PhD
The use of electronic Patient-Reported Outcome Measures (ePROMs) in palliative radiotherapy (PRT) offers an innovative approach to symptom monitoring and patient-centered care. This article summarises key findings from a PhD thesis on the development, implementation, and preliminary evaluation of the ePRomT diary, a digital tool developed to self-manage symptoms and help communication between patients and healthcare providers. The study highlights the feasibility, benefits, and challenges of ePROMs after palliative radiotherapy and their potential to improve palliative oncological care.
(BELG J MED ONCOL 2025;19(4):162–165)
Read moreBJMO - volume 18, issue 6, october 2024
H. Zaryouh PhD, J. De Waele PhD, I. De Pauw PhD, J.B. Vermorken MD, PhD, F. Lardon PhD, A. Wouters PhD
Head and neck squamous cell carcinoma (HNSCC) is often characterised by overexpression of the epidermal growth factor receptor (EGFR), which is associated with aggressive disease and poor prognosis. While the EGFR-targeting antibody cetuximab initially showed promise for the treatment of HNSCC, the development of therapeutic resistance has hindered its efficacy. Therefore, this PhD thesis focused on unravelling mechanisms underlying cetuximab resistance and developing strategic combination therapies to overcome this resistance in HNSCC. Literature review illustrated the importance of the PI3K/Akt pathway as a potential target, with studies indicating its role in resistance to anti-EGFR targeting agents. Protein phosphorylation profiling indicated that increased Akt1/2/3 phosphorylation seems to be characteristic for acquired cetuximab resistance in HNSCC cell lines. Interestingly, we observed synergy between cetuximab and Akt inhibitor MK2206 or PI3K inhibitor buparlisib in multiple cell lines with different sensitivity to cetuximab. Furthermore, the combination treatment of cetuximab plus buparlisib altered the tumour microenvironment, increasing tumour immunogenicity by triggering immunogenic cell death markers and reducing immunosuppression. This research underscores the potential for novel, rationally designed combination therapies to combat cetuximab resistance in HNSCC and suggests avenues for further exploration, including triple combination therapies involving immune checkpoint inhibitors, offering hope for improved outcomes in cancer therapy.
(BELG J MED ONCOL 2024;18(6):244-247)
Read moreBJMO - 2024, issue 4, june 2024
V. Depoorter PhD, K. Vanschoenbeek PhD, L. Decoster MD, PhD, C. Kenis RN, PhD, F. Verdoodt PhD, H. Wildiers MD, PhD
Oncology care is tumour-centric by tradition, but especially in older patients, a more holistic approach is needed that takes into account each aspect of the patient’s health status and not just the tumour. Identifying areas of vulnerability with geriatric screening (GS) and/or geriatric assessment (GA) is crucial in providing patient-oriented and multidisciplinary care that is tailored to the patient’s general health status. The results of GS/GA allow the treating physician to apply clinical judgment based on an estimate of biological age to optimise cancer treatment decisions. The use of GS/GA is, however, not yet widespread in Belgian oncology practice so further evidence on what GS/GA results can contribute, particularly regarding long-term outcomes, was needed to further stimulate the systematic implementation. This study specifically aimed to explore the association between the GS (with Geriatric 8 or G8) / GA and long-term outcomes using linked clinical and population-based data from a cohort of older patients with cancer. It was demonstrated that older patients with an abnormal G8 score at cancer diagnosis had a significantly lower 10-year overall survival compared to patients with a normal G8 score. Furthermore, patients with an abnormal baseline G8 score displayed higher healthcare utilisation across primary care, hospital care, and residential care in the three years after cancer diagnosis. In deceased patients with an abnormal baseline G8 score, functional and cognitive impairment identified with GA at cancer diagnosis was associated with less specialised palliative care use in the last three months of life.
(BELG J MED ONCOL 2024;18(4):160–3)
Read moreBJMO - volume 18, issue 1, february 2024
T. Muilwijk MD
Patients with high-risk non-muscle-invasive bladder cancer (NMIBC) are at risk for disease progression, which encompasses significant treatment-related and cancer-specific morbidity and mortality. Therefore, stratification of patients is important to identify patients that are at risk for progression of disease. However, current risk stratification lacks accuracy to identify which patients will progress and ultimately succumb to their disease. This PhD thesis focused on identifying prognostic biomarkers to optimise risk stratification of patients with NMIBC. The tumour immune microenvironment (TIME) and markers for basal and luminal differentiation were analysed using an immunohistochemical (IHC) panel in patients with high-risk T1 NMIBC. Interestingly, we identified fibroblast activation protein-α (FAP) expression as a prognostic marker for progression in highrisk NMIBC. FAP is a marker for cancer-associated fibroblasts (CAF), which play an important role in the TIME.
(BELG J MED ONCOL 2024;18(1):29–32)
Read moreBJMO - volume 17, issue 7, november 2023
G. Devos MD, PhD, G. De Meerleer MD, PhD, W. Everaerts MD, PhD, S. Joniau MD, PhD
The aim of this thesis was to assess the impact of intensive hormonal therapy prior to radical prostatectomy in high-risk prostate cancer (PCa) in order to optimise the outcome of this patient population. Next, the recurrence patterns of PCa patients with prostate-specific antigen (PSA) relapse following localised therapy were assessed using novel imaging techniques such as prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT). Moreover, the safety and oncological outcomes of PCa men with oligometastatic recurrence (1–5 lesions) following localised therapy treated with metastasis-directed therapy were investigated. Lastly, the safety and efficacy of radium-223 in men with PSA relapse following localised therapy without visible lesions on PSMA PET/CT were assessed.
(Belg J Med Oncol 2023;17(7):267–70)
Read moreBJMO - volume 17, issue 6, october 2023
M. Baudelet PhD, F. Duprez MD, PhD, M. De Bodt PhD, G. Van Nuffelen PhD
Dysphagia is a common and widely reported complication during and after radiotherapy (RT) for head and neck cancer (HNC), affecting quality of life (QoL). Research concerning the use of prophylactic swallowing exercises (PSE) is growing, and positive effects on muscle composition, swallowing function and QoL have already been demonstrated. However, low adherence to PSE undermines the beneficial effects. The PRESTO program, an optimised, patient-tailored and evidence-based prophylactic swallowing program augmented with adherence-improving measures was, therefore, developed. Different general but also more specific methods to increase adherence were considered. The way the PSE program was delivered depended on the treatment group (paper-, app- or therapist-supported PSE). After implementing PRESTO in 148 oropharyngeal cancer patients treated at four different hospitals in Flanders, results showed that the adherence towards PSE was the highest in the therapist-supported group. Face-to-face therapy may, thus, solve the problem of low adherence rates. In addition, it was observed that only patients practising at a high frequency (≥75% of the prescribed exercises) would achieve positive effects of the PSE on swallowing function and muscle strength. The results of this PhD research are clinically relevant and contribute to better supportive care in patients with HNC.
(Belg J Med Oncol 2023;17(6):236–8)
Read moreBJMO - volume 17, issue 4, june 2023
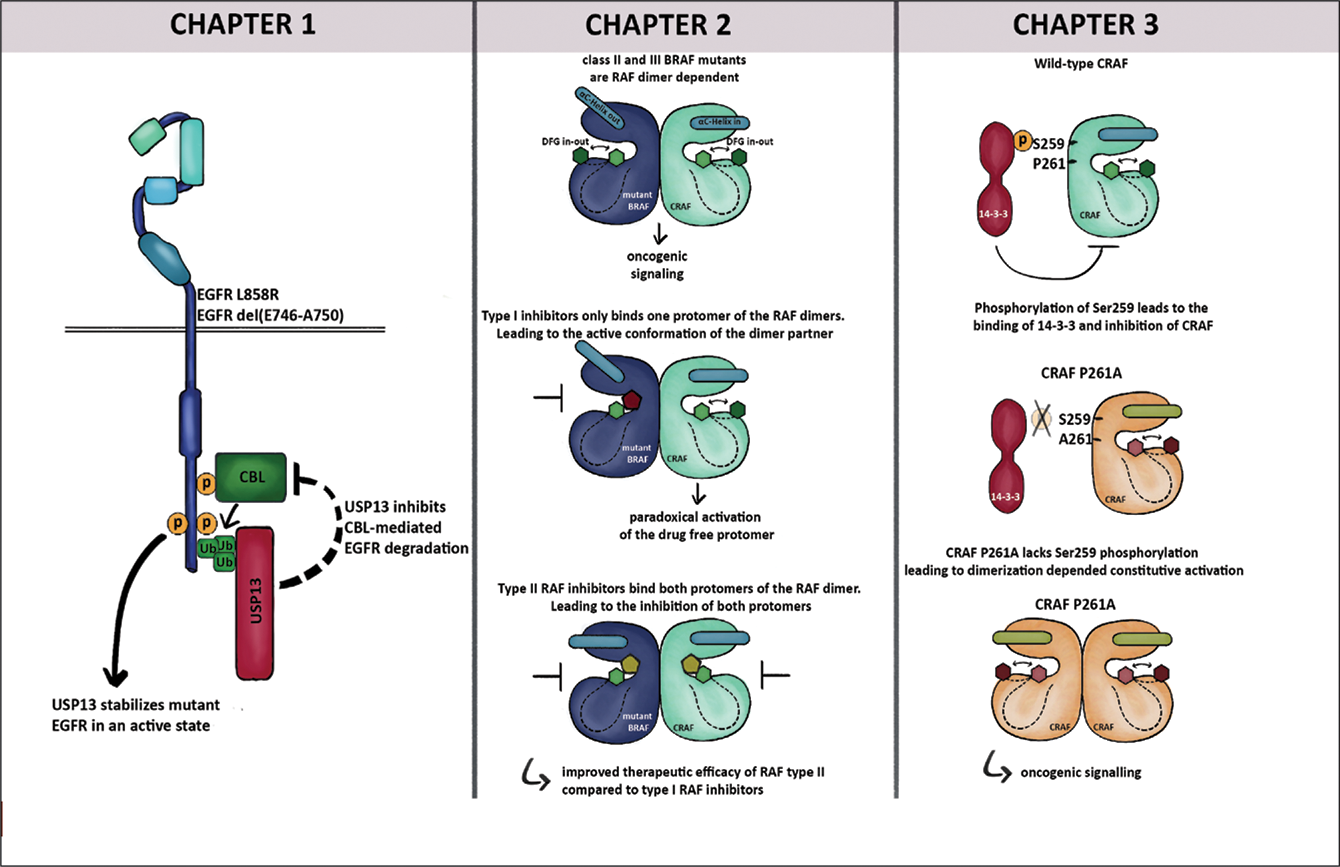
P. Giron PhD, J. De Grève MD, PhD
GRAPHICAL ABSTRACT
(Belg J Med Oncol 2023;17(4):132–4)
Read more