
Editor’s pick of Hans Wildiers, MD, PhD, medical oncologist, University hospital Leuven
Oncologists treating early breast cancer patients are often confronted with the dilemma of avoiding undertreatment while minimising the risk of overtreatment. In recent years, three biomarker-based clinical trials (MINDACT, TAILOR-x and Rx-PONDER) have provided strong evidence of safely foregoing chemotherapy in older postmenopausal women with luminal breast cancer at high clinical risk but low genomic risk.
Earlier screening mammography, new locoregional treatment modalities and the wider adoption of adjuvant systemic therapies have led to a reduction in breast cancer (BC) mortality. As the medical management of these patients takes on a personalised approach, oncologists treating ‘early BC’ should aim to avoid undertreatment, as well as overtreatment. Until recently, it was unclear which populations of BC patients may benefit from adjuvant chemotherapy (CT), and who may safely forego it. Now, three large precision medicine clinical trials provide insight into the dilemma, enabling better adjuvant chemotherapy tailoring via gene expression signatures in women with luminal BC at high clinical risk but low genomic risk.
In 2019-2020, the MINDACT trial established the clinical utility of the MammaPrint® commercial gene expression signature, while the TAILOR-x and Rx-PONDER trials did the same for the Oncotype-Dx gene expression signature. Although all three clinical trials aimed to identify a patient population who could safely forego adjuvant chemotherapy, the populations enrolled by these trials were relatively diverse. TAILOR-x enrolled only node-negative patients, whilst 79% of MINDACTs population were node-negative and 21% had 1-3 positive nodes. Conversely, Rx-PONDER enrolled exclusively node-positive patients (1-3 nodes). TAILOR-x was designed to establish whether endocrine therapy (ET) alone was not inferior to ET + CT in patients with a mid-range recurrence score (RS) of 11-25. The primary endpoint was invasive disease-free survival (iDFS) at five years. Similarly, the primary endpoint of MINDACT focussed on the 5-year distant metastases-free survival (DMFS) in clinical high risk (48% node positive, 58% with tumour size >2 cm, 29% with histological grade 3) but genomic low risk patients receiving only ET. Finally, Rx-PONDER aimed to demonstrate a positive interaction of chemotherapy benefit with increasing RS. Figure 1 details the comparative design of the three trials.
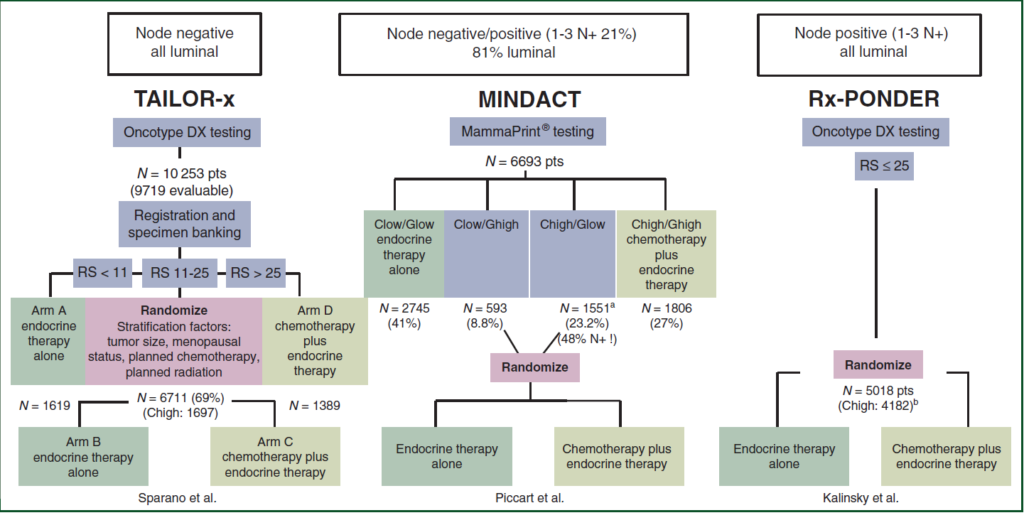
Figure 1: Comparison of study design between TAILOR-x, MINDACT and Rx-PONDER
For TAILOR-x, at a median follow-up of 9 years, the primary endpoint of non-inferiority for iDFS was met. The hazard ratio for the endocrine groups versus the chemo-endocrine group was 1.08 (95%CI: 0.94-1.24) and the distant recurrence rate was approximately 5%, irrespective of chemotherapy. In MINDACT, at a median follow-up of 8.7 years, a 5-year DMFS of 95% was observed in clinical high genomic low patients assigned to receive no chemotherapy, meeting the primary endpoint of a 5-year DMFS >92%. Conversely however, Rx-PONDER reported a benefit with the addition of CT to ET (HR[95%CI]: 0.81[0.67-0.98]). However, the trial failed to demonstrate a positive interaction of CT benefit with increasing RS, the primary hypothesis. Importantly, Rx-PONDER uncovers a positive interaction between chemotherapy benefit and menopausal status, with the CT benefit confined exclusively to premenopausal women.
Interestingly, when the clinical high, genomic low risk definition provided by the Adjuvant! Online algorithm used in MINDACT is applied to the results of TAILOR-x and Rx-PONDER, all three trials provide comparable results, with a clear survival benefit observed with adjuvant CT in premenopausal women (or women aged 50 years or less), whilst postmenopausal women (or women above the age of 50 years) derive no benefit from the addition of CT (Figure 2). For the younger women, there is a 43%-46% relative reduction in the rate of primary endpoints events (distant recurrence, DMFS or IDFS), translating into absolute benefits of 5%-6%, which are clinically meaningful for most patients and clinicians.
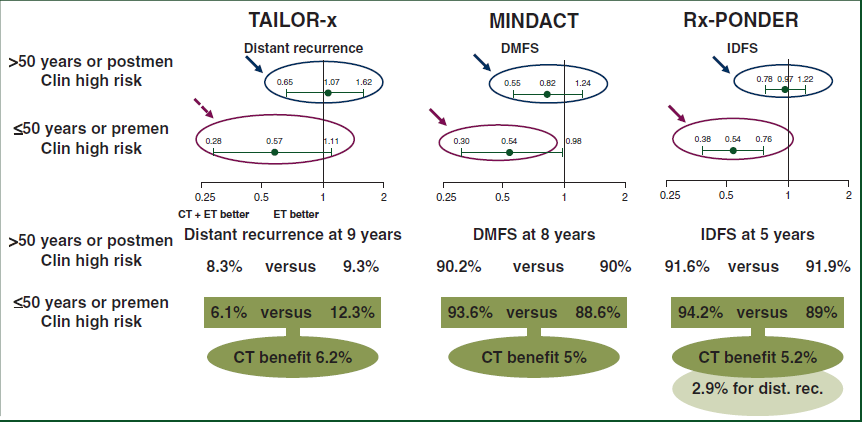
Figure 2: Results of all 3 trials when the Adjuvant! Online definition of clinical high, genomic low risk is applied
In light of this analysis, the convergence of these results further solidifies the clinical utility of the Oncotype-Dx and MammaPrint® gene expression tools as solutions to identify older postmenopausal women who have a high clinical risk (up to three positive nodes), but low genomic risk (RS <26 or ‘low risk’ 70 gene signature), who can safely forego adjuvant chemotherapy.
Reference
Piccart MJ, Kalinsky K, Gray R, et al. Gene expression signatures for tailoring adjuvant chemotherapy of luminal breast cancer: stronger evidence, greater trust. Annals of Oncol. 2021;32(9):1077-82.